2.1 Atoms, Ions, and Molecules: The Building Blocks of Chemical Evolution
After you complete this section, you should be able to . . .
-
Analyze the relationship between atomic structure and how atoms interact in simple molecules.
An atom is the smallest identifiable unit of matter. Just four types of atoms—hydrogen (H), carbon (C), nitrogen (N), and oxygen (O)—make up 96 percent of all matter found in organisms today. Many of the molecules found in your cells contain thousands, or even millions, of these atoms bonded together. But early in Earth’s history, H, C, N, and O existed only in simple substances such as water and carbon dioxide, which contain just three atoms apiece.
Two questions are fundamental to understanding how these simple substances could have evolved into the more complex molecules found in living cells:
-
What are the physical structures of hydrogen, carbon, nitrogen, and oxygen atoms?
-
What are the structures of the simple molecules—water, carbon dioxide, and others—that served as the building blocks of chemical evolution?
The focus on structure follows from one of the most central themes in biology: Structure affects function. To understand how a molecule affects your body or the role it played in chemical evolution, you have to understand how it is put together.
Basic Atomic Structure
Figure 2.1a shows a simple way of depicting the structure of an atom, using hydrogen and carbon as examples. Extremely small particles called electrons orbit an atomic nucleus made up of larger particles called protons and neutrons. Every element except hydrogen has one or more neutrons in its nucleus. Figure 2.1b provides a sense of scale at the atomic level.
Figure 2.1 Parts of an Atom
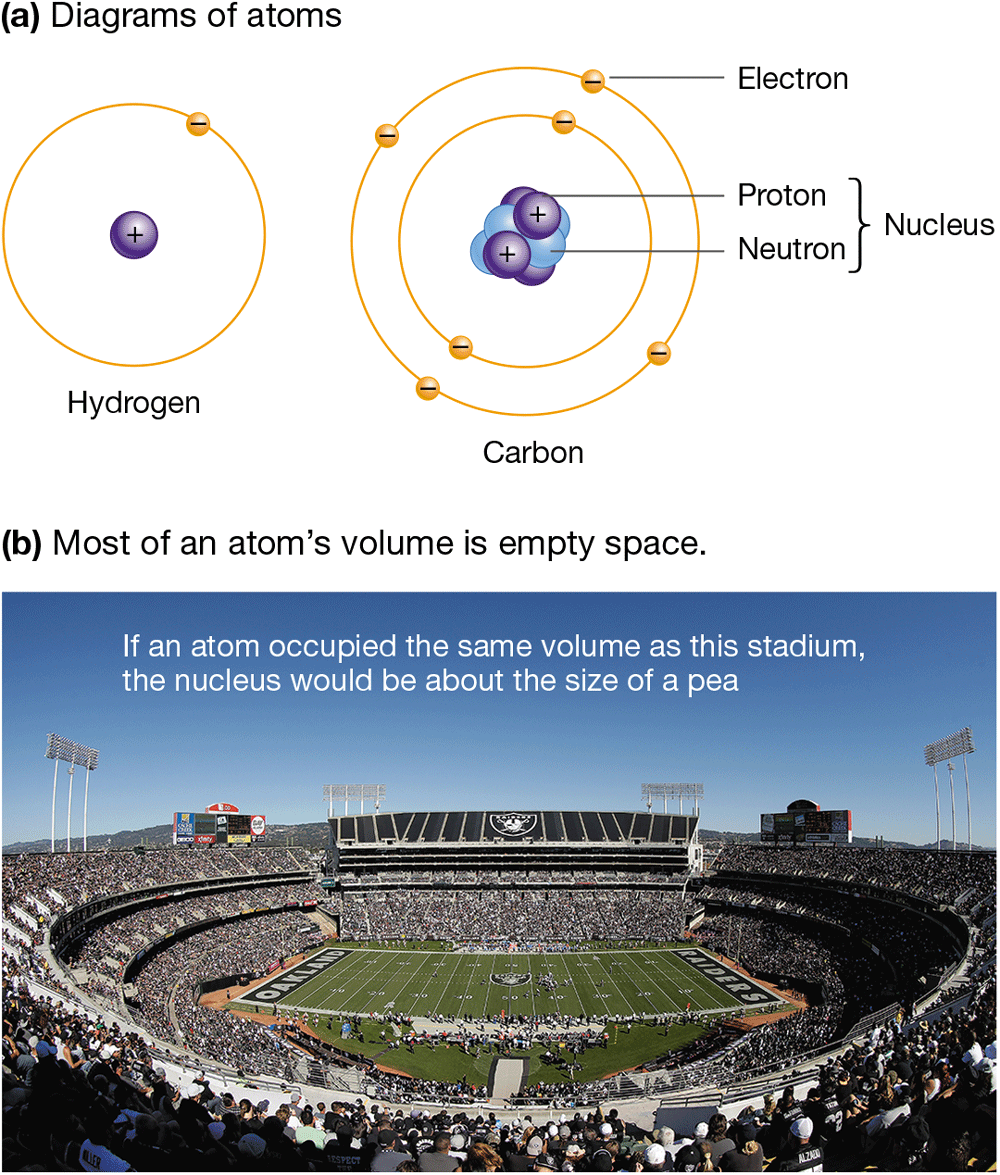
A simplified model of an atom with its nucleus, made up of protons and neutrons—or a single proton in the case of hydrogen—surrounded by orbiting electrons. In reality, electrons are not evenly spaced, nor do they orbit the nucleus in concentric circles; their actual orbits are complex.
Protons have a positive electric charge , neutrons are electrically neutral, and electrons have a negative electric charge . When the number of protons and the number of electrons in an atom are the same, the charges balance and make the entire atom electrically neutral.
Figure 2.2 shows a segment of the periodic table of the elements. Elements are defined as substances that consist entirely of a single type of atom. Notice that each atom of a given element contains a characteristic number of protons, called its atomic number. The atomic number is written as a subscript to the left of an element’s symbol in Figure 2.2. The sum of the protons and neutrons in an atom is called its mass number and is written as a superscript to the left of its symbol.
Figure 2.2 A Portion of the Periodic Table
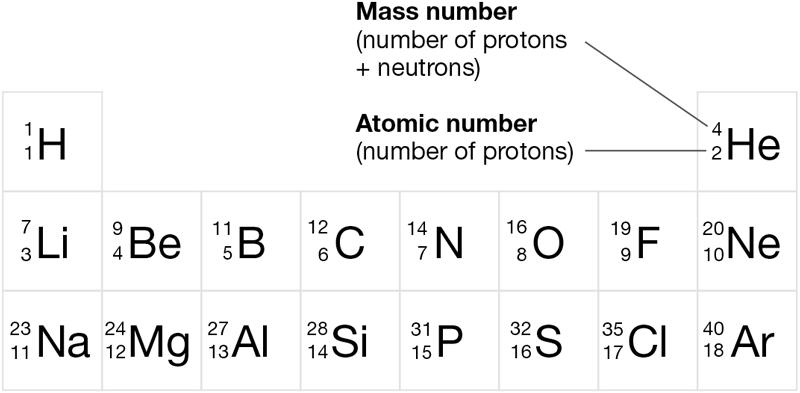
Each element has a unique atomic number and is represented by a unique one- or two-letter symbol. The mass numbers given here are the most common for each element. (Appendix B provides a complete periodic table.)
Although the masses of protons, neutrons, and electrons can be measured in grams, the numbers involved are so small that biologists prefer to use a special unit called the dalton (Da). This unit of measure was named after John Dalton, who was responsible for formulating modern atomic theory in the seventeenth century. The masses of protons and neutrons are virtually identical and are routinely rounded to 1 Da each. The mass of an electron is so small that it is normally ignored. So, the mass of an atom is equal to its mass number.
The number of protons in an element does not vary—if the atomic number of an atom changes, then it is no longer the same element. The number of neutrons present in an element can vary, however. Forms of an element with different numbers of neutrons are known as isotopes (literally “equal-places,” because all the forms occupy the same position in the periodic table).
Isotopes of the same element have different masses because they have different numbers of neutrons. All atoms of the element carbon have 6 protons, for example, but naturally occurring isotopes of carbon can have 6, 7, or even 8 neutrons, giving them masses of 12, 13, or 14 Da, respectively. The atomic weight of an element is an average of all the masses of the naturally occurring isotopes based on their abundance in nature. This is why the atomic weight of an element is often slightly different from its mass number. For example, carbon’s atomic weight is 12.01 rather than 12, which reflects that while the most abundant isotope of carbon has 6 neutrons and a mass of 12 daltons ( ), there are also less abundant isotopes with greater atomic weights.
Most common isotopes are stable, but not all. For example, , with 8 neutrons, represents an unstable radioactive isotope. Its nucleus will eventually decay and release energy (in the form of radiation). When decays, one of its neutrons changes into a proton, converting to the stable isotope of nitrogen, with 7 protons and 7 neutrons. Timing of decay is specific to each radioactive isotope, a fact that has been very useful in estimating the dates of key events in Earth’s history (Ch. 22, Section 22.2).
To understand how the structures of atoms differ, take a moment to study Figure 2.3. This chart highlights in blue the elements that are most abundant in living cells. The elements C, H, N, O, P, and S make up over 99 percent of the atoms in your body.
Figure 2.3 The Atomic Structure of the First 18 Elements
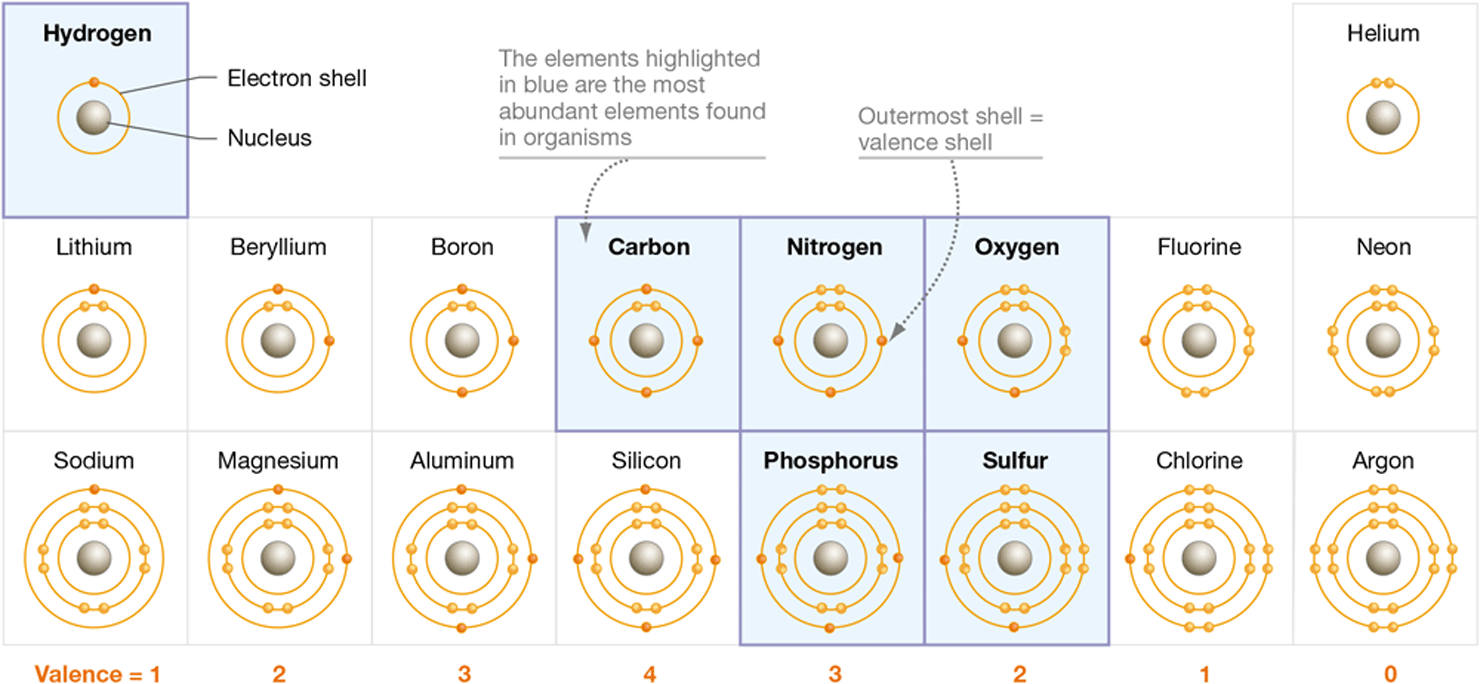
The most abundant elements in organisms are highlighted in blue.
Figure 2.3
Watch Animation: Structure of the Atomic Nucleus
The arrangement of electrons around the nucleus is key to understanding how different elements behave.
-
Electrons move around atomic nuclei in specific regions called orbitals. Each orbital can hold up to two electrons (i.e., a pair).
-
Orbitals are grouped into levels called electron shells.
-
Electron shells are numbered 1, 2, 3, and so on, to indicate their relative distance from the nucleus. Smaller numbers are closer to the nucleus.
-
Each electron shell contains a specific number of orbitals. Each orbital in a shell is loaded with one electron before any orbital is filled with a second, paired electron.
-
The electrons of an atom fill the innermost shells first, before filling outer shells.
Now focus on the outermost shell of each element. This is the atom’s valence shell. The electrons found in this shell are referred to as valence electrons. Note that in each of the highlighted elements, the outermost electron shell is not full—it contains at least one orbital with an unpaired valence electron. The number of unpaired valence electrons varies among elements. Carbon, for example, has four valence electrons, all unpaired. Oxygen has six valence electrons; four are paired, two are not. The number of unpaired electrons found in an atom’s valence shell is referred to as its valence. Carbon’s valence is four, oxygen’s is two.
The observations just made are significant because an atom is most stable when its valence shell is filled. One way that valence shells can be filled is through the formation of chemical bonds—attractions that bind atoms together. When two atoms share electrons, the chemical bond is called a covalent bond, and the connected atoms are termed a molecule.
Watch Animation: Electron Arrangement
How Does Covalent Bonding Hold Molecules Together?
To understand how atoms can become more stable by making covalent bonds, consider hydrogen. The hydrogen atom has just one electron, which resides in a valence shell that can hold two electrons.
Because it has an unpaired valence electron, the hydrogen atom does not have a full valence shell and is not very stable. But when two atoms of hydrogen come into contact, their two electrons become shared by their two nuclei as shown in Figure 2.4. This in effect gives each atom a filled outer shell. Together, the hydrogen atoms are more stable than the two individual hydrogen atoms.
Figure 2.4 Covalent Bonds Result from Electron Sharing
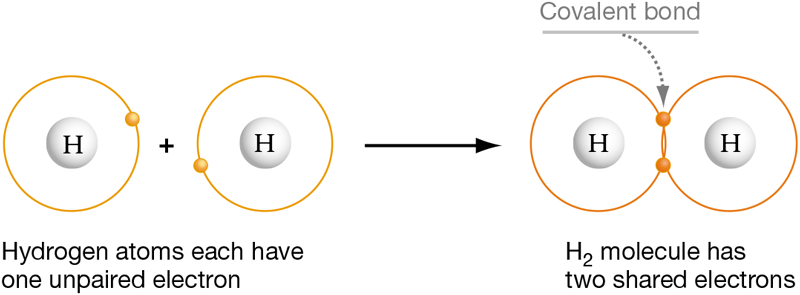
When two hydrogen atoms form a covalent bond, their unpaired valence electrons are shared by each nucleus.
Watch Animation: Covalent Bonds
Shared electrons “glue” atoms together into molecules. In the case of two hydrogen atoms, the bonded atoms form a single molecule of hydrogen, written as H―H or .
Nonpolar and Polar Covalent Bonds
In Figure 2.5a, the covalent bond between hydrogen atoms is represented by a dash and the electrons are drawn as dots halfway between the two nuclei. This depiction shows that the electrons are shared equally between the two hydrogen atoms, resulting in a covalent bond that is symmetrical.
Figure 2.5 Electron Sharing and Bond Polarity

Electrons in a covalent bond can be (a) shared equally, resulting in nonpolar bonds, or (b) shared unequally, resulting in polar bonds. Delta symbols and associated with polar covalent bonds refer to partial charges that arise owing to unequal electron sharing.
It’s important to note, though, that the electrons participating in a covalent bond are not always shared equally between the atoms involved. This may occur in substances called compounds, in which atoms of different elements are bonded together. When atoms of different elements form a bond, they may pull shared electrons toward their nuclei with varying strengths. Chemists call this property of an atom its electronegativity.
What is responsible for an atom’s electronegativity? It’s a combination of two things—the number of protons in the nucleus and the distance between the nucleus and the valence shell. If you return to the periodic table in Figure 2.3 and move your finger along a full row from left to right, you will be moving toward elements that increase in number of protons and in electronegativity (ignoring those with full outer shells in the far right column). Each row in the table represents a shell of electrons. As your finger moves down the table, it passes over elements with more shells and less electronegativity. In Figure 2.3, fluorine would have the highest electronegativity and sodium would have the lowest.
Oxygen, which has eight protons and only two electron shells, is among the most electronegative of all elements. It attracts covalently bonded electrons more strongly than does any other atom commonly found in organisms. Nitrogen, which has one fewer proton, has a somewhat lower electronegativity than oxygen. Sulfur, carbon, hydrogen, and phosphorus, in turn, have relatively low and approximately equal electronegativities. Thus, the electronegativities of the six most abundant elements in organisms are related as follows:
Because carbon and hydrogen have approximately equal electronegativity, the electrons in a C—H bond are shared equally or symmetrically. A bond that involves equally shared electrons is called a nonpolar covalent bond. In contrast, asymmetric sharing of electrons results in a polar covalent bond. The electrons in a polar covalent bond spend most of their time close to the nucleus of the more electronegative atom. Why is this important?
Watch Animation: Nonpolar and Polar Molecules
Polar Bonds Produce Partial Charges on Atoms
To understand the consequences of differences in electronegativity and the formation of polar covalent bonds, consider the water molecule. Water consists of an oxygen atom bonded to two hydrogen atoms, and is written . As Figure 2.5b illustrates, electrons involved in the covalent bonds in water are not shared equally but are held much more tightly by the oxygen nucleus than by the hydrogen nuclei. Hence, both bonds in a water molecule, between each of the hydrogen atoms and the oxygen atom, are polar covalent bonds.
Here’s the key observation: Because electrons are shared unequally in each bond, they spend more time near the oxygen atom, giving it a partial negative charge, and less time near the hydrogen atoms, giving them a partial positive charge. These partial charges are symbolized by the lowercase Greek letter delta (𝛿), together with a plus or minus sign.
As you will see (Section 2.2), the partial charges on water molecules—due simply to the difference in electronegativity between oxygen and hydrogen—are one of the primary reasons that life exists.
Ionic Bonding, Ions, and the Electron-Sharing Continuum
Ionic bonds are similar in principle to covalent bonds, but instead of being shared between two atoms, the electrons in ionic bonds are completely transferred from one atom to the other. The electron transfer occurs because it gives each of the two resulting atoms a full valence shell.
A sodium atom (Na), for example, has three electron shells with a lone electron in its valence shell. Sodium atoms tend to lose an electron, leaving them with a full second shell—a much more energetically stable arrangement (Figure 2.6a). The atom that results has a net electric charge of , because it has one more proton than it has electrons.
Figure 2.6 Ion Formation and Ionic Bonding
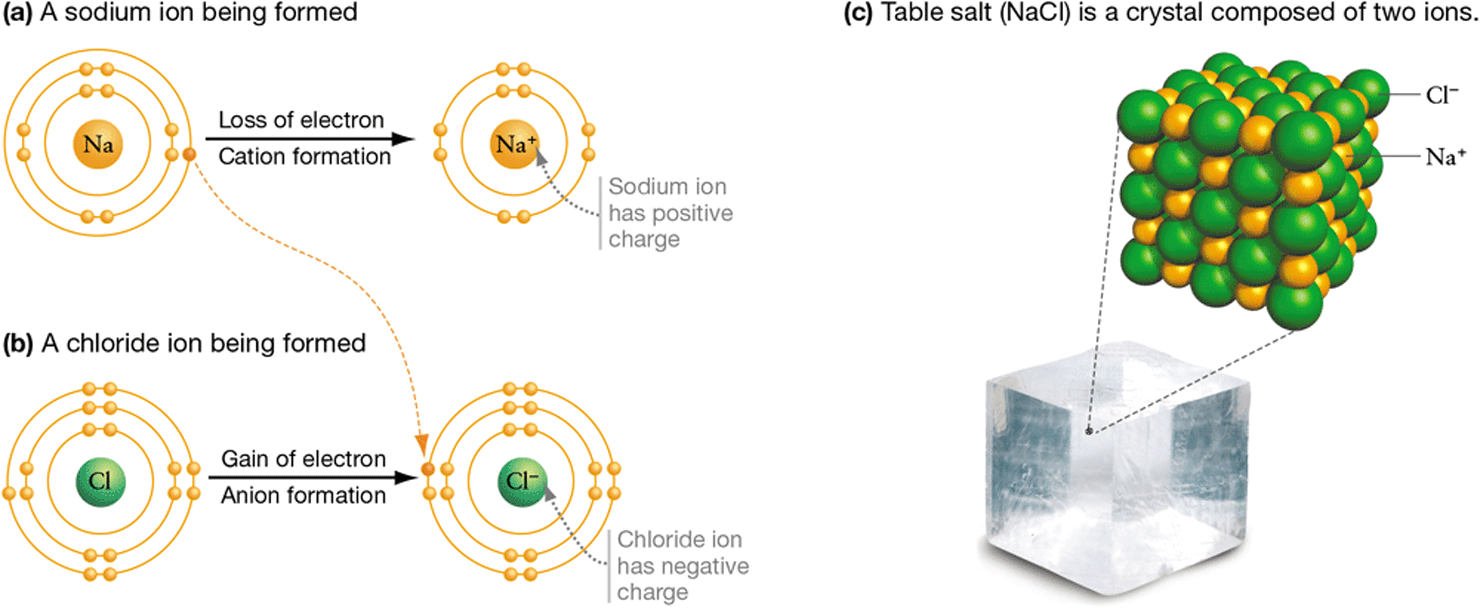
The sodium ion ( ) and the chloride ion ( ) are stable because they have full valence shells. In table salt (NaCl), sodium and chloride ions pack into a crystal structure held together by electrical attraction between their positive and negative charges.
Watch Animation: Ionic Bonds
An atom or molecule that carries a full charge, rather than the partial charges that arise from polar covalent bonds, is called an ion. The sodium ion is written and, like other positively charged ions, is called a cation (pronounced KAT-eye-un).
Chlorine atoms (Cl), in contrast, tend to gain an electron, filling their outermost shell (Figure 2.6b). The resulting ion has a net charge of −1, because it has one more electron than protons. This negatively charged ion, or anion (pronounced AN-eye-un), is written and is called chloride.
When sodium cations and chloride anions combine to form sodium chloride (NaCl, common table salt), they pack into a crystal structure held together by electrical attraction between opposite charges on the ions (Figure 2.6c). The electrical attraction is so strong that salt crystals are difficult to break apart.
Based on the previous discussions of covalent and ionic bonds, Figure 2.7 illustrates an important general observation concerning the role of electrons. The degree to which electrons are shared in chemical bonds forms a continuum from equal sharing in nonpolar covalent bonds to unequal sharing in polar covalent bonds to the transfer of electrons in ionic bonds. Most of the compounds that are present in living organisms are formed from either nonpolar or polar covalent bonds.
Figure 2.7 The Electron-Sharing Continuum
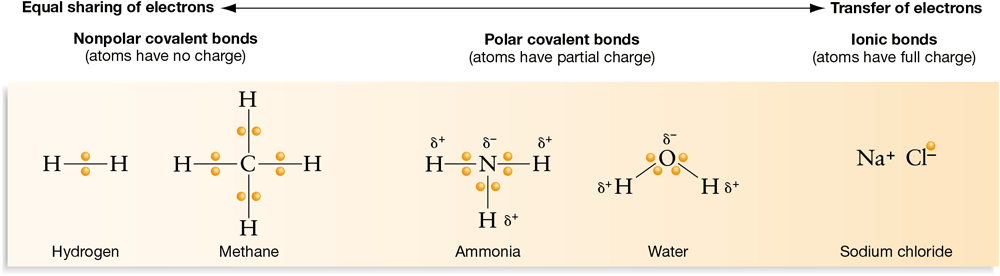
The degree of electron sharing in chemical bonds can be thought of as a continuum, from equal sharing in nonpolar covalent bonds to complete electron transfer in ionic bonds.
Figure 2.7
Let’s look next at how covalent bonds hold atoms together in molecules.
Some Simple Molecules Formed from C, H, N, and O
Look back at the periodic table in Figure 2.3 and count the number of unpaired electrons in the valence shells of carbon, nitrogen, oxygen, and hydrogen. Each unpaired electron in a valence shell can make up half of a covalent bond. It should make sense to you that a carbon atom can form a total of four covalent bonds; nitrogen can form three; oxygen can form two; and hydrogen, one.
When each of the four unpaired electrons of a carbon atom covalently bonds with a hydrogen atom, the molecule that results is written and is called methane (Figure 2.8a). Methane is the most common molecule found in natural gas. When a nitrogen atom’s three unpaired electrons bond with three hydrogen atoms, the result is , or ammonia. Similarly, an atom of oxygen can form covalent bonds with two atoms of hydrogen, resulting in a water molecule ( ). As Figure 2.4 showed, a hydrogen atom can bond with another hydrogen atom to form hydrogen gas .
Figure 2.8 Unpaired Electrons in the Valence Shell Participate in Covalent Bonds
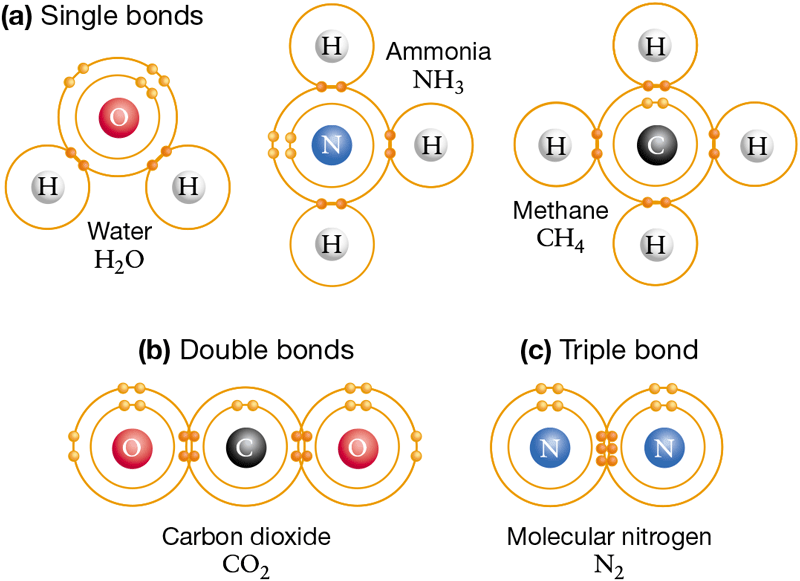
Covalent bonding is based on sharing of electrons in the outermost shell. Covalent bonds can be (a) single, (b) double, or (c) triple.
Figure 2.8
Atoms with more than one unpaired electron in the valence shell can also form double bonds or triple bonds. Figure 2.8b shows how carbon forms double bonds with oxygen atoms to produce carbon dioxide . Triple bonds result when three pairs of electrons are shared. Figure 2.8c shows the structure of molecular nitrogen , which forms when two nitrogen atoms establish a triple bond.
Note that in each of the molecules in Figure 2.8, the single, double, or triple covalent bonds have the effect of giving each atom a full outer shell. Each nitrogen atom in , for example, has one unshared pair of electrons and three shared electron pairs to fill its valence shell with a total of eight electrons.
The Geometry of Simple Molecules
In many cases, the overall shape of a molecule dictates how it behaves. In chemistry and in biology, function is based on structure.
The shapes of the simple molecules you’ve just learned about are governed by the geometry of their bonds. The position of these bonds results from the repulsive forces between the negative charges in shared and unshared electron pairs in the valence shell.
-
Nitrogen and carbon dioxide have linear structures (see Figure 2.8). There are only two atoms in , so the molecule can only be linear. The three atoms in are linear because the electrons in the two bonds repel one another and are thus 180° apart, which maximizes the distance between them.
-
Methane has a tetrahedral structure (Figure 2.9a). The tetrahedron forms because the repulsive forces between electrons push the four bonds as far apart as they can get, such that each bond to the central carbon atom is 109.5° away from its neighboring bonds.
-
Water ( ) has a planar (flat) structure, with a bond geometry that is bent rather than linear (Figure 2.9b). Why? The electrons in the four orbitals of oxygen’s valence shell repulse each other, just like the electrons in the bonds in methane do. But in water, two of the orbitals in the central oxygen atom are filled with unshared electron pairs, which push the bonds closer together than what is observed in methane’s bonds. The result is a flat, V-shaped molecule with bonds that are 104.5° apart.
Figure 2.9 The Geometry of Methane and Water
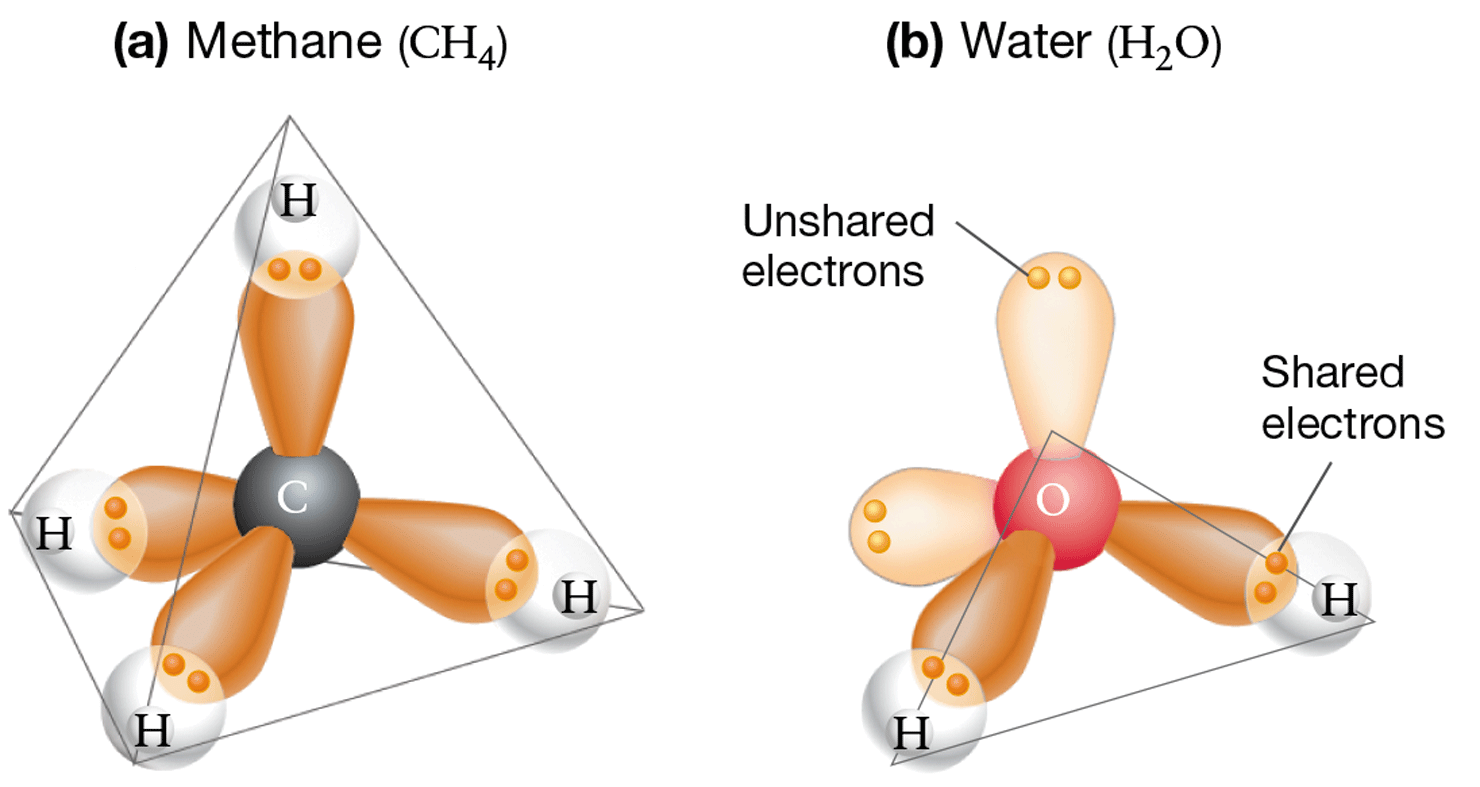
Later in this chapter you will explore how water’s shape, in combination with the partial charges on the oxygen and hydrogen atoms, makes it the most important molecule on Earth (Section 2.2).
Representing Molecules
Molecules can be represented in a variety of increasingly complex ways—only some of which reflect their actual shape. Each method has advantages and disadvantages (see BioSkills 14).
-
Molecular formulas are compact, but don’t contain a great deal of information—they indicate only the numbers and types of atoms in a molecule (Figure 2.10a).
-
Structural formulas indicate which atoms in a molecule are bonded together. Single, double, and triple bonds are represented by single, double, and triple dashes, respectively. Structural formulas also indicate geometry in two dimensions (Figure 2.10b). This method is useful for planar molecules such as water and .
-
Ball-and-stick models take up more space than structural formulas, but provide information on the three-dimensional shape of molecules and often indicate the relative sizes of the atoms involved (Figure 2.10c).
-
Space-filling models are more difficult to read than ball-and-stick models but more accurately depict the relative sizes of atoms and their spatial relationships (Figure 2.10d).
Figure 2.10 Molecules Can Be Represented Several Ways
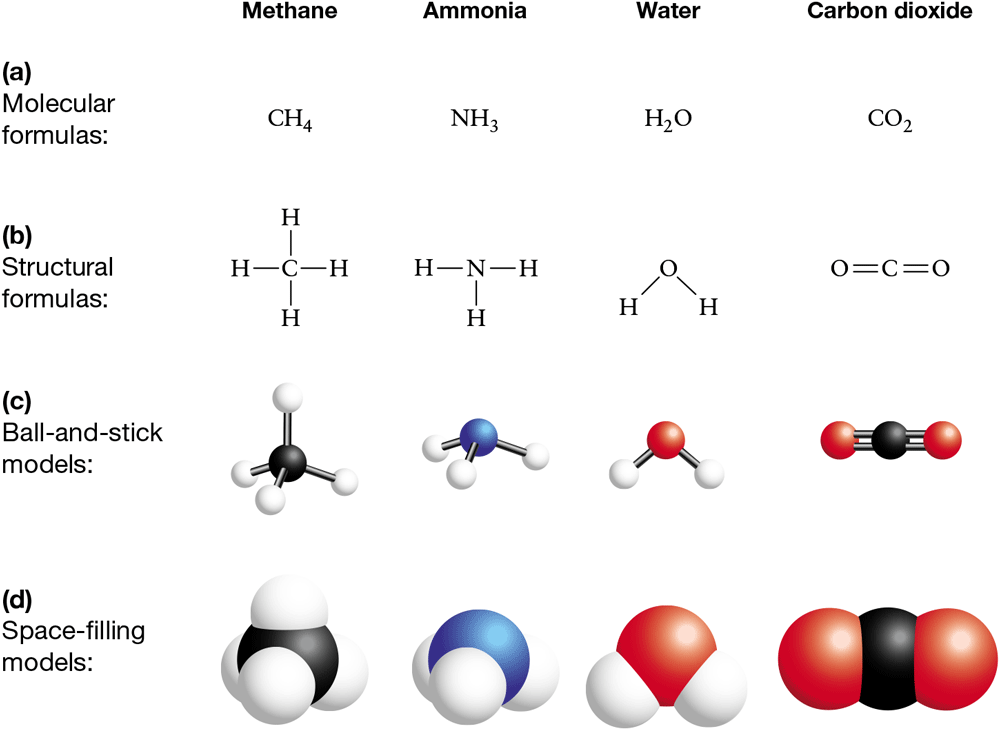
Each method of representing a molecule has particular advantages.
In both ball-and-stick and space-filling models, biologists use certain colors by convention to represent certain atoms. A black ball, for example, always symbolizes carbon.
Some of the small molecules you’ve just learned about are found in volcanic gases, the atmospheres of nearby planets, and in deep-sea hydrothermal vents like those shown in the photograph at the start of this chapter. Based on these observations, researchers propose that they were important components of Earth’s ancient atmosphere and oceans. If so, then they could have provided the building blocks for chemical evolution. The question is: How did these simple building blocks combine to form more complex products early in Earth’s history?
Researchers postulate that most of the critical reactions in chemical evolution occurred in an aqueous, or water-based, environment. To understand what happened and why, let’s delve into the properties of water and then turn to analyzing the reactions that triggered chemical evolution.